Dr. Pasricha is the Vice Chair of Medicine for Innovation and Commercialization and Professor of Medicine and Neurosciences at the Johns Hopkins University School of Medicine and Professor of Innovation Management in the Johns Hopkins Carey School of Business.
After a series of calamitous news cycles a few years ago, the gastroenterological community and
the various stakeholders appear to have entered a new phase of complacency with the belief that
changes in the design of the new series of duodenoscopes (mainly in the form of removable caps
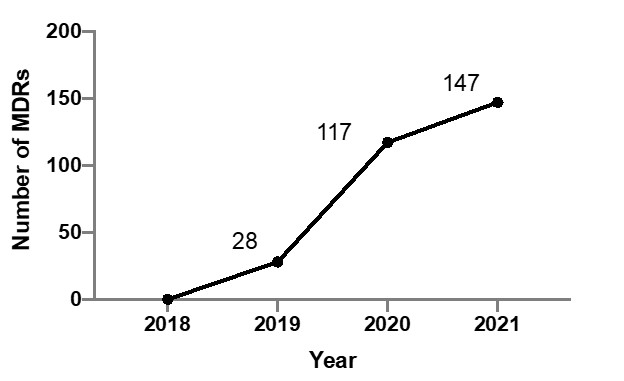
by Olympus, Pentax or Fuji) or the advent of novel disposable duodenoscopes (Boston Scientific, Ambu) will soon put these problems in the rear-view mirror. However, the MAUDE (Manufacturer and User Facility Device Experience) database maintained by the US Food and Drug Administration (FDA), which mandates manufacturers to post medical device reports (MDRs) of suspected device-associated deaths, serious injuries and malfunctions recently showed a more than 5-fold increase in new duodenoscope related MDRs through April, 2021 (Figure 1), compared to 2019.1
It can be argued that endoscopists are not reporting problems with conventional endoscopes with
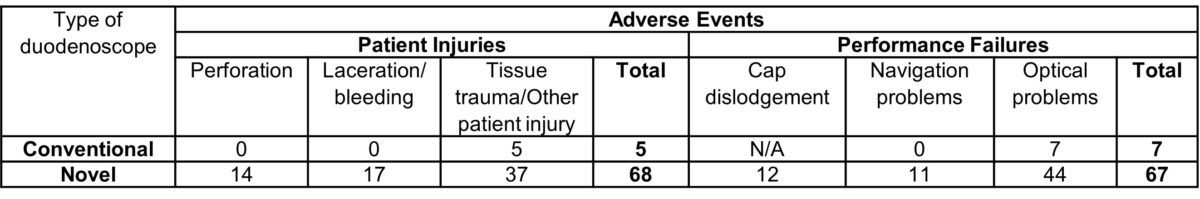
the same diligence as with the new models. Perforations do occur at a low frequency with conventional duodenoscopes but are almost always due to factors other than endoscope design. For the latter, the FDA reporting mandates applies equally to all devices in the field (regardless of time in the field) and hospitals have 10 days to report after an adverse event with a device.2 In this regard, examination of the MDRs for all duodenoscopes in all of 2021, the first full year when all the novel duodenoscopes were being used side-by-side with the conventional ones may be useful. The results are summarized in Table 1.
Table 1. Medical Device Reports on adverse events for duodenoscopes in the MAUDE database for 2021 (accessed in February 2022)
Considering that the proportion of cases using the newer duodenoscopes in 2021 may at best be
1-10% of the total number of ERCP cases (estimated to be about 700,000 per year), the incidence of perforations with the novel endoscopes ranges from 0.2 to 2 per 1000, compared to the reported 1.2 per 1000 with conventional endoscopes. 3 Manufacturers have actually begun to acknowledge these design issues with instructional videos on how to avoid injury from the same. 4 Until we can confidently determine the actual risk of injuries, it is therefore our collective responsibility as physicians and patient advocates to take these reports seriously. Examination of the same database for whether the new innovations are living up to their promise of reducing contamination may be viewed with more certainty as these are being reported diligently for all endoscope designs. Contaminations were reported in 250 conventional duodenoscopes and 88 of the new ones (presumably this only applies to those with removable caps). Again, when correcting for the frequency of use, new duodenoscopes fared much worse in their ability to prevent contamination (with relative risks of 3-fold or higher, assuming 10% of all cases were performed by such scopes). This is not particularly surprising because the removable caps do not necessarily prevent contamination of the elevator or in the upstream channels and the new duodenoscopes have attachment points for the caps that present additional crevasses that must be manually cleaned to avoid biomatter retention.
While recognizing that the data is still evolving, it is clear that the new designs do not represent a
breakthrough in preventing contamination. Patients therefore have a right to this information,
given that there may be an as yet unproven greater risk for injury. Technological innovations can provide a better, safer path from the dangerous cascade described above, but they must be smarter in design to prevent infections without sacrificing the safety and high-performance standards of the conventional duodenoscopes. At least one novel device (ScopeSeal®) has been approved by the FDA for this purpose and has the potential for addressing this vexing problem in a simple, effective and environmentally friendly manner (in full disclosure, the author is a consultant for the manufacturer of this device). 5 However, clinical trials are still awaited. Hopefully, other innovations may be forthcoming as well and we can truly envision and progress to a safer future for patients undergoing procedures with duodenoscopes.
Disclosures
Dr. Pasricha has served as a consultant and owns stocks in GI Scientific.